Despite revolutionary advancements in cancer care through immunotherapy, particularly immune checkpoint inhibitors (ICIs), a significant proportion of patients do not achieve durable responses, frequently due to tumors evolving sophisticated mechanisms of immune escape. New research sheds light on a previously underestimated systemic process of immune suppression, revealing how cancer cells actively disseminate immunosuppressive signals throughout the body via tiny extracellular vesicles, and identifying a common medication, statins, as a potential disruptor of this critical pathway. This discovery not only provides a deeper understanding of immunotherapy resistance but also proposes an immediately actionable strategy for improving patient outcomes.
The advent of immune checkpoint inhibitors, particularly those targeting the programmed death-1 (PD-1) and programmed death-ligand 1 (PD-L1) axis, has fundamentally transformed the landscape of oncology. By disarming the "brakes" on the immune system, these agents enable T-cells to recognize and eliminate malignant cells, leading to remarkable, long-lasting remissions in a subset of patients across various cancer types. This success has fueled intense optimism and a surge in research aimed at broadening the applicability and efficacy of such treatments. However, the sobering reality remains that for the majority of individuals, these therapies either fail to induce a response (primary resistance) or lose effectiveness over time (acquired resistance). This persistent challenge underscores the urgent need to unravel the intricate mechanisms by which cancers evade the host immune response, extending beyond the immediate tumor microenvironment.
Traditional investigations into immunotherapy resistance often focused on intrinsic tumor characteristics, such as mutational burden, antigen presentation machinery, or the composition of immune cells infiltrating the tumor. While these factors are undeniably crucial, a growing body of evidence suggests that cancers employ more pervasive, systemic strategies to dampen immune activity throughout the entire organism. This broader perspective shifts the focus from localized immune evasion to mechanisms that establish a suppressive immunological environment distal to the primary tumor. Among these emerging mechanisms, the role of small extracellular vesicles (sEVs), nanoparticles secreted by virtually all cells, has garnered significant attention. These sEVs, laden with proteins, lipids, and nucleic acids, act as critical mediators of intercellular communication, capable of influencing recipient cells at distant sites. In the context of cancer, sEVs released by tumor cells can carry immunosuppressive cargo, effectively "educating" or "corrupting" immune cells throughout the body, thereby creating a fertile ground for immune escape and contributing to systemic resistance.
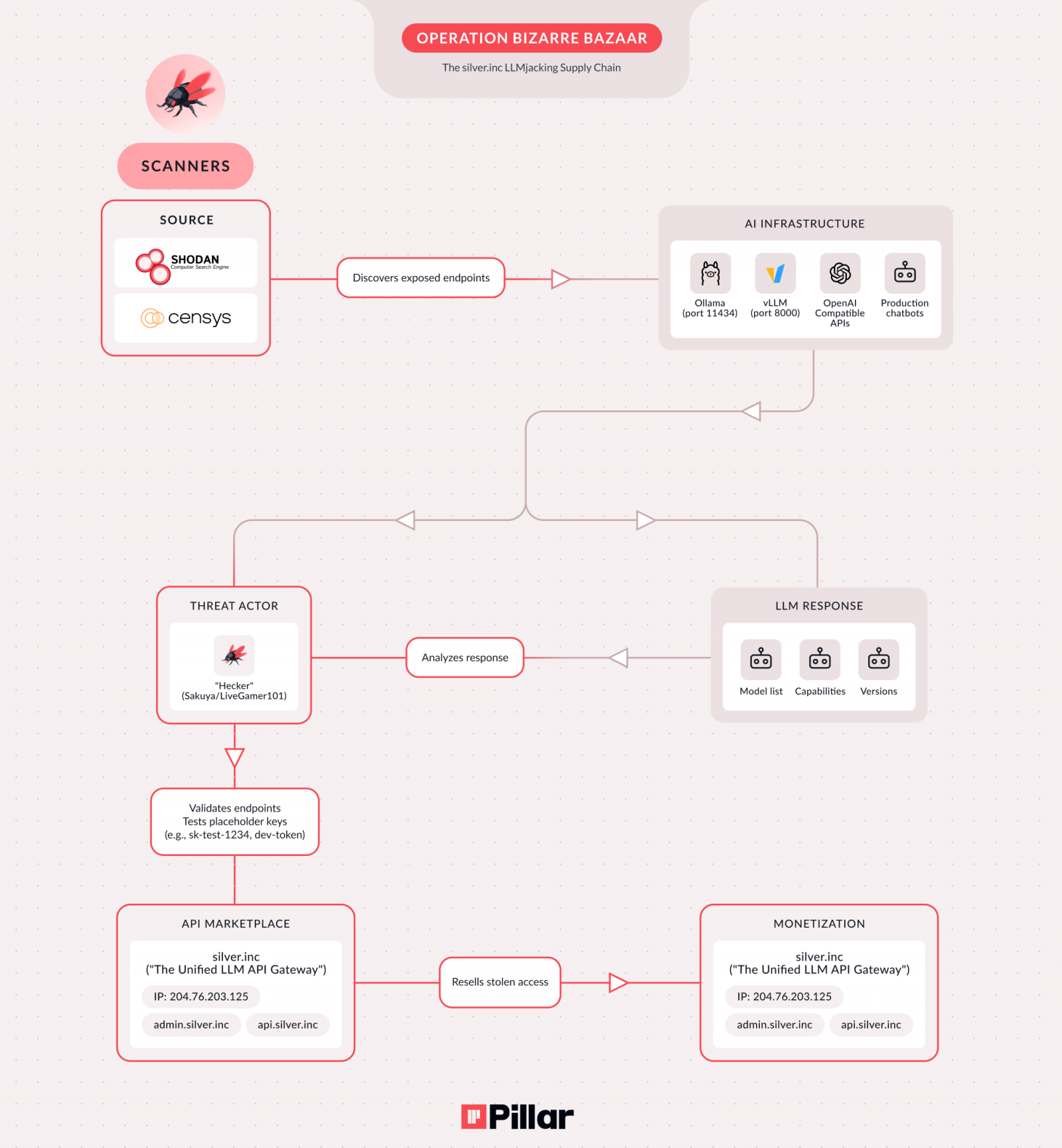
Deciphering the Packaging Mechanism of Immunosuppressive Cargo
A pivotal area of inquiry has been to understand precisely how key immunosuppressive molecules, such as PD-L1, are packaged into these sEVs and subsequently disseminated. PD-L1, a transmembrane protein expressed on cancer cells and certain immune cells, interacts with PD-1 on T-cells, leading to T-cell inactivation. While the role of membrane-bound PD-L1 is well-established, the presence of PD-L1 within sEVs suggests an additional, potent mechanism for immune suppression, allowing the tumor to project its immunosuppressive influence far beyond its immediate physical boundaries.
A dedicated research team, comprising scientists from Fujita Health University in Japan, led by Professor Kunihiro Tsuchida, in collaboration with experts from Tokyo Medical University Hospital and Tokyo Medical University, embarked on an ambitious quest to elucidate the molecular machinery governing the selective loading of PD-L1 into sEVs. The central hypothesis driving their investigation, published in the esteemed journal Scientific Reports, revolved around a critical unanswered question: how is PD-L1 specifically sorted and sequestered into these tiny vesicles, enabling its release into the systemic circulation to undermine immune responses? Addressing this fundamental knowledge gap was considered paramount for developing strategies to counteract this pervasive form of immune evasion.
UBL3: A Novel Regulator of PD-L1 Trafficking
Leveraging a comprehensive and multi-faceted experimental approach, encompassing advanced molecular and cell biology techniques, sophisticated biochemical and pharmacological assays, analysis of patient-derived clinical samples, and robust bioinformatics methodologies, the research team made a groundbreaking discovery. They identified ubiquitin-like 3 (UBL3) as a crucial molecular determinant controlling the selective channeling of PD-L1 into sEVs. This finding represents a significant advancement in understanding the complex regulatory networks governing protein trafficking and immune modulation in cancer.
Their detailed investigations revealed that PD-L1 undergoes a previously unrecognized post-translational modification, distinct from the canonical ubiquitination process, involving UBL3. This novel modification occurs through the formation of a disulfide bond, specifically at the cysteine 272 residue located within the cytoplasmic tail of the PD-L1 protein. Post-translational modifications are critical regulatory events that profoundly influence protein function, stability, localization, and interactions. The identification of UBL3-mediated disulfide bonding as a specific modification for PD-L1’s sEV packaging pathway highlights the intricate molecular fine-tuning employed by cancer cells to orchestrate immune suppression.
Further rigorous experimentation unequivocally confirmed the central role of UBL3. When UBL3 expression was experimentally increased in cancer cells, a dramatic and quantifiable rise in the amount of PD-L1 packaged into sEVs was observed, strikingly, without any concomitant change in the total intracellular levels of PD-L1. Conversely, when UBL3 levels were suppressed or genetically reduced, there was a pronounced decrease in PD-L1 loading into vesicles and its subsequent release from the cells. These robust gain-of-function and loss-of-function experiments provided compelling evidence, establishing UBL3 as a direct and indispensable orchestrator in directing PD-L1 into the sEV secretory pathway.
Statins: An Unexpected Interceptor of Immune Escape
Perhaps the most immediately impactful finding of the study emerged when the research team systematically screened for existing pharmacological agents that could potentially interfere with this newly identified UBL3-driven mechanism. Their efforts led to a remarkable discovery: statins, a class of drugs widely prescribed globally for their cholesterol-lowering properties, were found to potently inhibit the UBL3-mediated modification of PD-L1. All clinically utilized statins tested in the study, without exception, demonstrated a significant reduction in UBL3 activity, a consequent decrease in PD-L1 modification, and a sharp curtailment of PD-L1 sorting into sEVs.
Crucially, these therapeutic effects were observed at very low drug concentrations, well within the range routinely achieved in patients receiving statin therapy, and importantly, without inducing any discernible toxic effects on the cellular viability or function. This finding immediately elevated the translational potential of the discovery, suggesting that a safe, established medication could be repurposed to enhance immunotherapy.
The clinical relevance of these in vitro and ex vivo findings was further substantiated by examining patient samples. Analysis of blood samples collected from individuals diagnosed with non-small cell lung cancer (NSCLC) revealed a compelling correlation: among patients exhibiting high PD-L1 expression within their tumors, those who were concurrently receiving statin medication displayed significantly lower levels of PD-L1-containing sEVs in their bloodstream compared to patients not on statins. This direct human evidence provides a powerful validation of the proposed mechanism and underscores its potential clinical applicability. Moreover, comprehensive bioinformatic analysis, integrating genetic and expression data, indicated that the combined expression levels of UBL3 and PD-L1 were significantly associated with overall survival outcomes in lung cancer patients, further reinforcing the clinical importance of this newly delineated regulatory pathway.
Transformative Implications for Cancer Treatment
Collectively, these groundbreaking results offer a profound mechanistic explanation for a significant proportion of immune checkpoint inhibitor failures. They illuminate a previously hidden mechanism by which cancer cells actively propagate immunosuppressive signals through the systemic circulation via extracellular vesicles. By deploying PD-L1-laden sEVs, tumors effectively extend their immunological reach, weakening the host immune response far beyond their immediate anatomical location, thereby establishing a systemic environment conducive to immune evasion and resistance.
The serendipitous link between this critical immune escape pathway and statins carries immense therapeutic implications. Statins are among the most widely prescribed medications worldwide, renowned for their favorable safety profile, affordability, and extensive clinical experience. This combination of attributes significantly accelerates the potential for translating these preclinical findings into tangible clinical benefits. The prospect of repurposing an existing, well-tolerated drug to enhance the efficacy of cutting-edge cancer immunotherapies is exceptionally appealing, offering a rapid and cost-effective pathway to potentially improve patient outcomes. As articulated by the research team, "In the long term, this research may lead to more effective and accessible cancer immunotherapies. It could help more patients benefit from immune checkpoint treatments, improving survival and quality of life in real-world settings."
This study identifies vesicle-associated PD-L1 trafficking, driven by UBL3 modification, as a modifiable driver of immune escape. By disrupting this process, statins effectively reduce the levels of circulating immunosuppressive PD-L1, thereby potentially ‘re-sensitizing’ cancer cells and the systemic immune environment to the effects of immune checkpoint blockade. This opens a promising new avenue for tackling primary and acquired resistance to cancer immunotherapy. Incorporating statins into combination treatment strategies could offer a simple, scalable, and readily implementable approach to improve the clinical efficacy of immune checkpoint inhibitors.
Future research will undoubtedly focus on validating these findings in larger clinical cohorts, designing prospective clinical trials to evaluate the efficacy of statin-ICI combinations, and elucidating the optimal dosing and timing of statin administration in this context. Furthermore, understanding if this UBL3-PD-L1 axis plays a role in other cancer types and exploring whether other small molecules can more specifically target UBL3 modification could pave the way for a new class of adjuvant therapies. This discovery marks a significant leap forward, offering renewed hope for expanding the reach and impact of cancer immunotherapy, ultimately benefiting a greater number of patients grappling with this formidable disease.