Recent advancements in biomedical research have unveiled a novel approach to addressing the pervasive challenge of age-related gastrointestinal decline, demonstrating that a specialized form of immunotherapy, traditionally employed in oncology, possesses the remarkable capacity to facilitate the self-repair of the intestinal lining, thereby mitigating the physiological consequences of aging and injury. This groundbreaking discovery holds profound implications for enhancing healthspan and improving the quality of life for an aging global population, offering a potential therapeutic avenue for conditions ranging from nutrient malabsorption to chronic inflammation.
The human digestive system is a marvel of biological engineering, with its innermost surface, the intestinal epithelium, serving as a critical interface between the body and the external environment. This delicate, single-cell layer is indispensable for the selective absorption of nutrients, the exclusion of harmful pathogens and toxins, and the modulation of the immune system. Under optimal physiological conditions, the intestinal epithelium exhibits an extraordinary regenerative capacity, completely renewing itself approximately every three to five days through a meticulously orchestrated process involving stem cell proliferation and differentiation. However, this finely tuned mechanism is highly susceptible to disruption by a multitude of factors, most notably the natural aging process and exposure to exogenous stressors such as radiation therapy. As individuals age, the efficiency of epithelial renewal often diminishes, leading to compromised barrier function, increased permeability, and a heightened state of chronic inflammation within the gut lumen. This age-associated dysfunction can manifest as diminished tolerance to certain foods, inefficient nutrient uptake, and the development of conditions colloquially referred to as "leaky gut syndrome," which is characterized by the translocation of microbial products and antigens from the intestinal lumen into the systemic circulation, potentially contributing to systemic inflammation and a host of extra-intestinal pathologies.
The scientific community has long recognized the critical need for interventions that can restore or bolster intestinal integrity in the face of age-related degeneration or acute injury. A recent collaborative effort by researchers at a prominent biomedical research institution has now identified a potent strategy to invigorate this intrinsic repair mechanism. Their innovative approach leverages the sophisticated machinery of Chimeric Antigen Receptor (CAR) T-cell therapy, a powerful form of adoptive cell immunotherapy that has revolutionized the treatment landscape for certain hematological malignancies. By strategically re-engineering this therapeutic modality for application within the gastrointestinal tract, the investigators have opened a promising pathway toward future clinical applications aimed at ameliorating age-related intestinal decline and enhancing overall digestive health.
The Pervasive Influence of Cellular Senescence on Tissue Homeostasis
This transformative research builds upon a foundation of extensive prior work focused on cellular senescence, a fundamental biological process intrinsically linked to aging and various age-related pathologies. Senescent cells are distinct from actively dividing cells and terminally differentiated cells; they enter a state of irreversible cell cycle arrest but resist programmed cell death (apoptosis). Instead of being cleared from tissues, these persistent cells accumulate over time in various organs, including the gut. Far from being inert, senescent cells are metabolically active and exert detrimental effects on their microenvironment through the secretion of a complex array of pro-inflammatory cytokines, chemokines, growth factors, and proteases, collectively termed the senescence-associated secretory phenotype (SASP). The SASP contributes significantly to chronic low-grade inflammation, tissue remodeling, and the creation of an environment that impedes tissue regeneration and promotes dysfunction. The accumulation of these lingering, secretory cells has been causally implicated in the pathogenesis of numerous age-related conditions, encompassing metabolic disorders like type 2 diabetes, neurodegenerative diseases such as dementia, and various forms of organ fibrosis.
Previous investigations spearheaded by the research team had successfully engineered specific immune cells—namely, anti-uPAR CAR T cells—to selectively identify and eliminate senescent cells in preclinical models. The urokinase plasminogen activator receptor (uPAR) is a cell surface glycoprotein that, while present on some normal cells, is often significantly upregulated on the surface of senescent cells, rendering it an ideal target for selective clearance. In earlier studies, the targeted removal of these senescent cells in murine models led to substantial improvements in the animals’ metabolic health, underscoring the broad therapeutic potential of this senolytic strategy.
Repurposing CAR T-Cell Therapy for Intestinal Regeneration
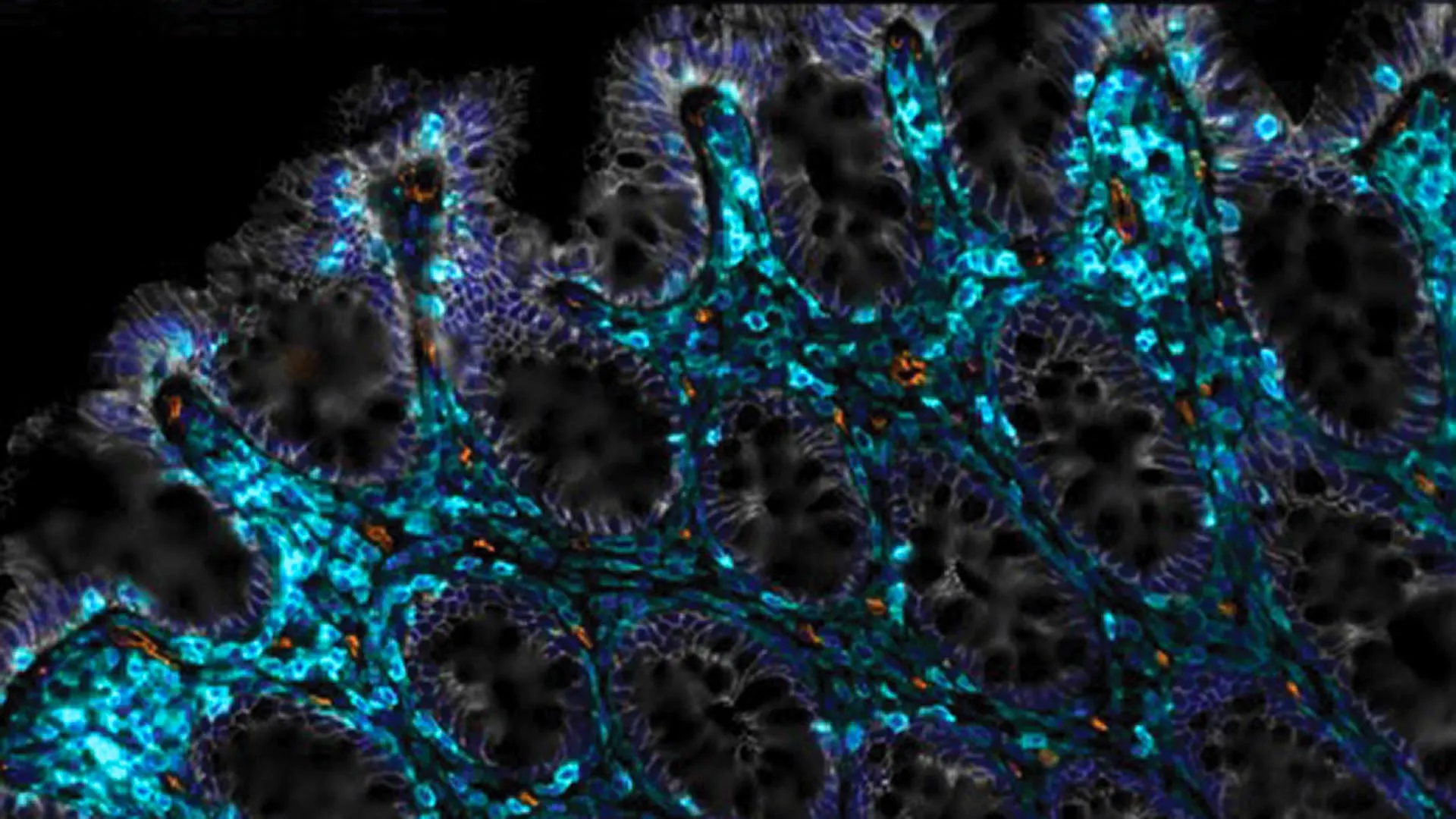
The logical progression of this research involved exploring whether the selective eradication of senescent cells could concurrently restore the intestine’s innate capacity for self-healing. To address this pivotal question, the research collaborators embarked on a series of rigorous experiments. They administered the specialized anti-uPAR CAR T cells directly into the intestines of both younger and older murine subjects. The ensuing observations were remarkably consistent and compelling, revealing significant physiological improvements across both age cohorts.
In the treated animals, there was a measurable enhancement in their ability to absorb nutrients, a critical function often compromised in aging guts. This improvement suggests a restoration of the epithelial barrier’s structural and functional integrity. Furthermore, the treated mice exhibited a marked reduction in intestinal inflammation, a key indicator of improved gut health and a common precursor to various gastrointestinal disorders. Perhaps most significantly, when challenged by irritation or injury, the intestinal epithelial lining in the CAR T-cell treated animals demonstrated a substantially accelerated capacity for regeneration and healing. This direct evidence of enhanced regenerative potential underscores the therapy’s ability to "jump-start" the intrinsic repair mechanisms of the gut, effectively reversing some of the age-related impediments to tissue homeostasis.
Mitigating Radiation-Induced Gut Damage: A Critical Application
Beyond the context of natural aging, the study also addressed a significant clinical challenge: radiation-induced enteropathy. Leaky gut syndrome and severe gastrointestinal dysfunction are particularly prevalent and debilitating complications among cancer patients undergoing pelvic or abdominal radiation therapy. To model this critical scenario, the research team exposed mice to controlled doses of radiation designed to induce damage to their intestinal epithelial cells. The results from this model were equally compelling. Mice that received the anti-uPAR CAR T-cell therapy demonstrated a far more effective and robust recovery from radiation-induced intestinal damage compared to their untreated counterparts.
A particularly noteworthy finding was the enduring efficacy of the treatment: a single administration of the CAR T-cell therapy continued to support healthier gut function for an impressive duration of at least one year. This sustained therapeutic effect is a crucial factor for clinical translation, suggesting that the intervention could offer long-term protection against chronic gut damage following acute injury, or provide sustained relief from age-related decline with infrequent dosing.
Further substantiating the translational relevance of their findings, the researchers also uncovered robust evidence that anti-uPAR CAR T cells actively encourage regeneration in human intestinal and colorectal cells ex vivo. While the precise molecular and cellular mechanisms underpinning this potent regenerative effect are still subjects of ongoing investigation, these findings provide strong preliminary validation for the therapeutic potential of this approach in human physiology. The broader significance of this work extends beyond targeted gut repair; it represents a fundamental step in expanding our understanding of how to effectively intervene in the aging process and promote tissue resilience in the elderly.
Implications and Future Directions: A New Frontier in Regenerative Medicine
The successful repurposing of CAR T-cell technology from its established oncological context to address age-related tissue degeneration marks a significant conceptual leap in regenerative medicine and geroscience. The implications of this research are multi-faceted and potentially transformative. For the elderly population, this therapy could offer a means to counteract the insidious decline in digestive efficiency, thereby improving nutrient absorption, reducing systemic inflammation, and potentially mitigating the risk of malnutrition and associated morbidities. The alleviation of chronic gut inflammation and the restoration of barrier function could have far-reaching effects on overall health, given the intricate connections between gut health and systemic immunity, metabolic regulation, and even neurological function (the gut-brain axis).
Beyond general aging, this approach holds promise for specific patient populations. Cancer patients receiving radiation therapy, who frequently suffer from severe and chronic gastrointestinal side effects, could benefit immensely from a therapy that accelerates healing and prevents long-term complications. Furthermore, this senolytic CAR T-cell strategy could potentially be explored for other conditions characterized by chronic inflammation and impaired tissue regeneration, such as inflammatory bowel diseases (Crohn’s disease, ulcerative colitis), post-surgical recovery involving intestinal anastomoses, and even graft-versus-host disease affecting the gut.
However, as with any pioneering therapeutic modality, substantial challenges lie ahead on the path to clinical translation. CAR T-cell therapies, even in oncology, are associated with potential adverse events, most notably cytokine release syndrome (CRS) and neurotoxicity. While these risks might differ in a non-cancer setting or with local administration, rigorous safety evaluations will be paramount. The manufacturing complexity and high cost associated with personalized cell therapies also present formidable hurdles for widespread accessibility. Future research will need to focus on optimizing CAR T-cell design to enhance specificity and safety, exploring alternative delivery mechanisms that are less invasive, and conducting comprehensive preclinical studies in larger animal models to fully characterize efficacy and long-term safety profiles.
Ultimately, the goal is to advance this technology towards human clinical trials, meticulously identifying appropriate patient cohorts and establishing clear endpoints for therapeutic success. This research paradigm, which targets fundamental mechanisms of aging such as cellular senescence, represents a crucial stride towards a future where maintaining robust physiological function and extending "healthspan"—the period of life spent in good health—becomes an achievable reality. The prospect of harnessing the body’s own immune system to reverse age-related decline in vital organs like the gut signifies a new era in precision medicine and offers profound hope for enhancing the health and well-being of an increasingly aging global demographic.







